Pro-Dense
Faster, Stronger, and Denser New Bone.*
Pro-Dense injectable regenerative graft has a unique triphasic resorption profile that provides an ideal environment for the direct deposition of bone by binding growth factors1 and by providing a slow-resorbing matrix that supports healing across the defect.
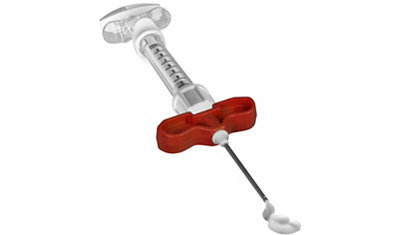
Pro-Dense was the first bone graft substitute indicated for the use in bone voids resulting from the resection of benign bone cysts in children 6 years and older. It delivers predicable bone regeneration with it’s triphasic resorption profile for faster, denser bone regeneration, stronger new bone, and a graft that remodels to normal bone.
*All claims based on a critically sized canine proximal humerus defect model. It is unknown how results from the canine modelcompare with clinical results in humans. Data on file at Stryker
- Growth factor binding based on in vitro data of BMP-2 and VEGF. Data on file.
AP-015803



